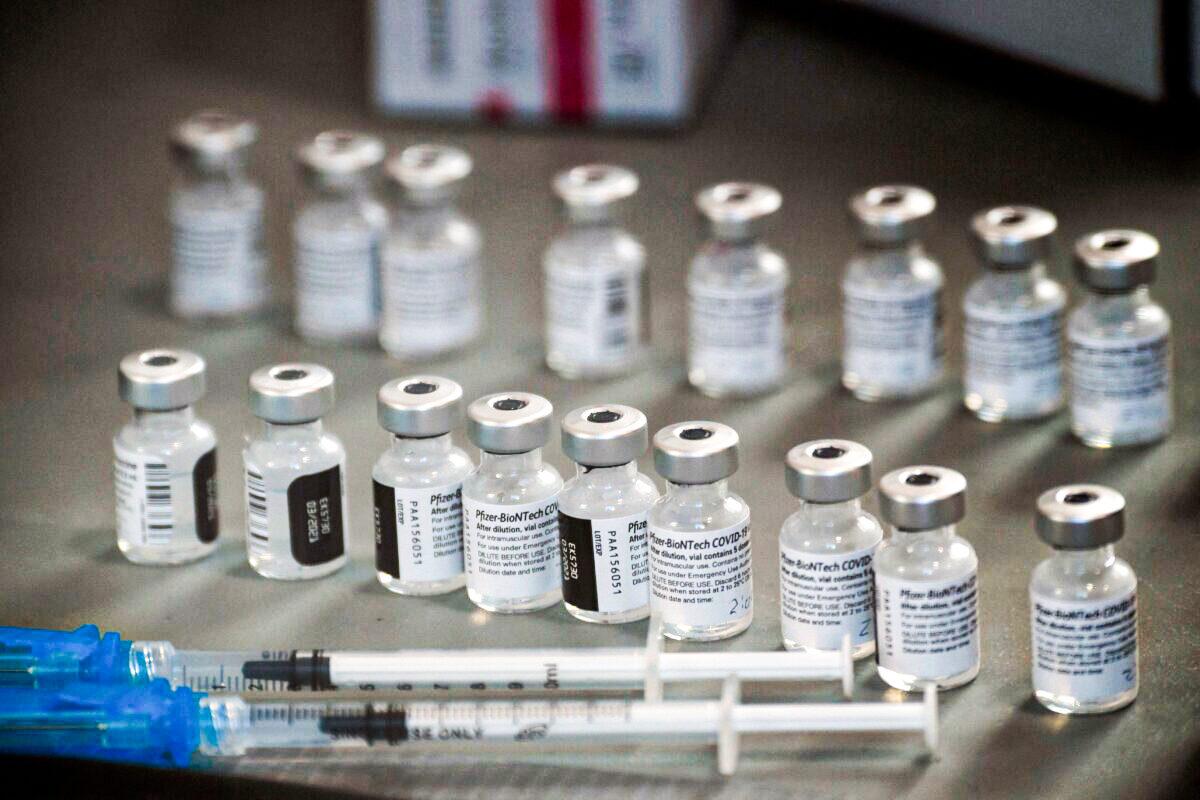
Syringes and vials of the Pfizer-BioNTech COVID-19 vaccine are prepared
to be administered at a drive-up vaccination site from Renown Health in
Reno, Nev., on Dec. 17, 2020. (Patrick T. Fallon/AFP via Getty Images)
COVID-19 cases among vaccinated seniors soared in 2021, according to newly disclosed data that was acquired by U.S. health agencies but wasn't presented to the public.
Humetrix Cloud Services was contracted by the U.S. military to analyze vaccine data. The company performed a fresh analysis as authorities considered in 2021 whether COVID-19 vaccine boosters were necessary amid studies finding waning vaccine effectiveness.
Humetrix researchers found that the proportion of total COVID-19 cases among the seniors was increasingly comprised of vaccinated people, according to the newly disclosed documents.
For the week ending on July 31, 2021, post-vaccination COVID-19 cases represented 73 percent of the cases among people 65 and older, the company found. The elderly were 80 percent fully vaccinated at the time.
Breakthrough infection rates were higher among those who were vaccinated early, the researchers found. They estimated that the rates were twice as high in those who had been vaccinated five to six months prior, when compared to people vaccinated three to four months before.
The breakthrough cases started in January 2021, according to the data.
Protection against hospitalization was also fading, researchers discovered.
In the week ending on July 31, 2021, 63 percent of the COVID-19 hospitalizations in seniors were among the fully vaccinated, according to the documents. The same pattern of weaker protection among people who were vaccinated early was found.
Researchers calculated that the vaccine effectiveness (VE) against infection was just 33 percent while the effectiveness against hospitalization had dropped to 57 percent.
Seniors who previously had COVID-19 and recovered were more likely to avoid hospitalization, the researchers also found. Risk factors included serious underlying conditions such as obesity and being in the oldest age group, or older than 85.
The cohort analysis was completed on 20 million Medicare beneficiaries, including 5.6 million seniors who received a primary series of a COVID-19 vaccine.
"Our observational study VE findings show a very significant decrease in VE against infection and hospitalization in the Delta phase of the pandemic for individuals vaccinated with either the Pfizer or Moderna vaccine for those 5–6 months post-vaccination vs. those 3–4 months post-vaccination," Dr. Bettina Experton, Humetrix's president and CEO, said in a Sept. 15, 2021, email to top U.S. Food and Drug Administration (FDA) officials.
Humetrix also found that among the beneficiaries, there had been 133,000 cases, 27,000 hospitalizations, and 8,300 intensive care admissions among the fully vaccinated since the start of the COVID-19 pandemic.
Dr. Experton disclosed that Humetrix shared the data with the U.S. Centers for Disease Control and Prevention (CDC) in August 2021.
"It would have been nice to know [the military] was conducting this prior to now. Also might have been nice for CDC to share the data," Dr. Peter Marks, one of the FDA officials, told colleagues in response.
"This is more worrisome than the other data we have in my opinion," Dr. Janet Woodcock, the FDA's acting commissioner at the time, said in reply.
"It is hard to see this as anything other than a failure of our health authorities to assess, share, make public, and act upon valuable, real-world data in the midst of a so-called pandemic," Del Bigtree, founder of the network, told The Epoch Times via email. "And without FOIA, the public likely would never be made aware of these failures which, of course, allows them to be perpetrated again and again."
The FDA and CDC declined to comment.
Dr. Francis Collins, the director of the U.S. National Institutes of Health at the time, wrote in a separate email obtained through FOIA that the results of the study provided "pretty compelling evidence that VE is falling 5–6 months post-vaccination for both infection and hospitalization for those over 65."
He added, "Even for those 3–4 months out, there is a trend toward worsening VE.”
The CDC, FDA, and National Institutes of Health didn't share the data with the public as they considered whether to clear and recommend COVID-19 vaccine boosters.
The CDC held a meeting with its vaccine advisers on Aug. 30, 2021. During the meeting, CDC officials went over emerging data on waning vaccine effectiveness, although the military study wasn't included.
The FDA held a similar meeting on Sept. 17, 2021. The CDC participated. The Humetrix analysis also wasn't presented during that meeting.
The presentation also included data from outside researchers and Israel that estimated the protection during the Delta era against infection ranged from 39 percent to 84 percent and that the effectiveness against hospitalization ranged from 75 to 95 percent.
The FDA ended up clearing a Pfizer booster for many Americans. The CDC advised most people to receive it. The agencies later expanded booster clearance and recommendations to virtually all Americans aged 5 and older, with Moderna's shot as another option. Authorities have since replaced the old shots because of their lack of durability and are preparing to roll out another slate of shots this fall.



No comments:
Post a Comment